Global S&T Development Trend Analysis Platform of Resources and Environment
| Interrupting immune-suppressing treatment can boost COVID vaccine response | |
| admin | |
| 2022-06-27 | |
| 发布年 | 2022 |
| 语种 | 英语 |
| 国家 | 英国 |
| 领域 | 资源环境 |
| 正文(英文) | 

A brief interruption in treatment for people who use immune-suppressing medicine can double the antibody response to COVID-19 booster vaccination. Experts from Imperial College London and the Universities of Nottingham and Oxford have studied the effect of temporarily stopping the use of the drug methotrexate for two weeks after a booster dose of a COVID-19 vaccine. The results of the major clinical trial will have implications for people on long-term immune suppressing medicine, who are among the millions of clinically vulnerable patients who were advised to “shield” during the pandemic. "[These results] have important implications for future vaccination strategy in this immunosuppressed patient group." Professor Rosemary Boyton Department of Infectious Disease Co-Lead Investigator Professor Rosemary Boyton, from Imperial’s Department of Infectious Disease, said: “This study is the first to report the effectiveness of a two-week interruption of an immunosuppressant drug called methotrexate immediately after COVID-19 booster vaccination. “Our results showed a doubling of antibody levels, an increase that was sustained at 12 weeks. This has important implications for future vaccination strategy in this immunosuppressed patient group.” Methotrexate is a commonly used immune-suppressing drug, with around 1.3 million people in the UK prescribed it for inflammatory conditions such as rheumatoid arthritis and skin conditions such as psoriasis. The drug is effective in controlling such conditions, but it also reduces the body’s ability to fight infection and generate good immune responses following flu and pneumonia vaccination. 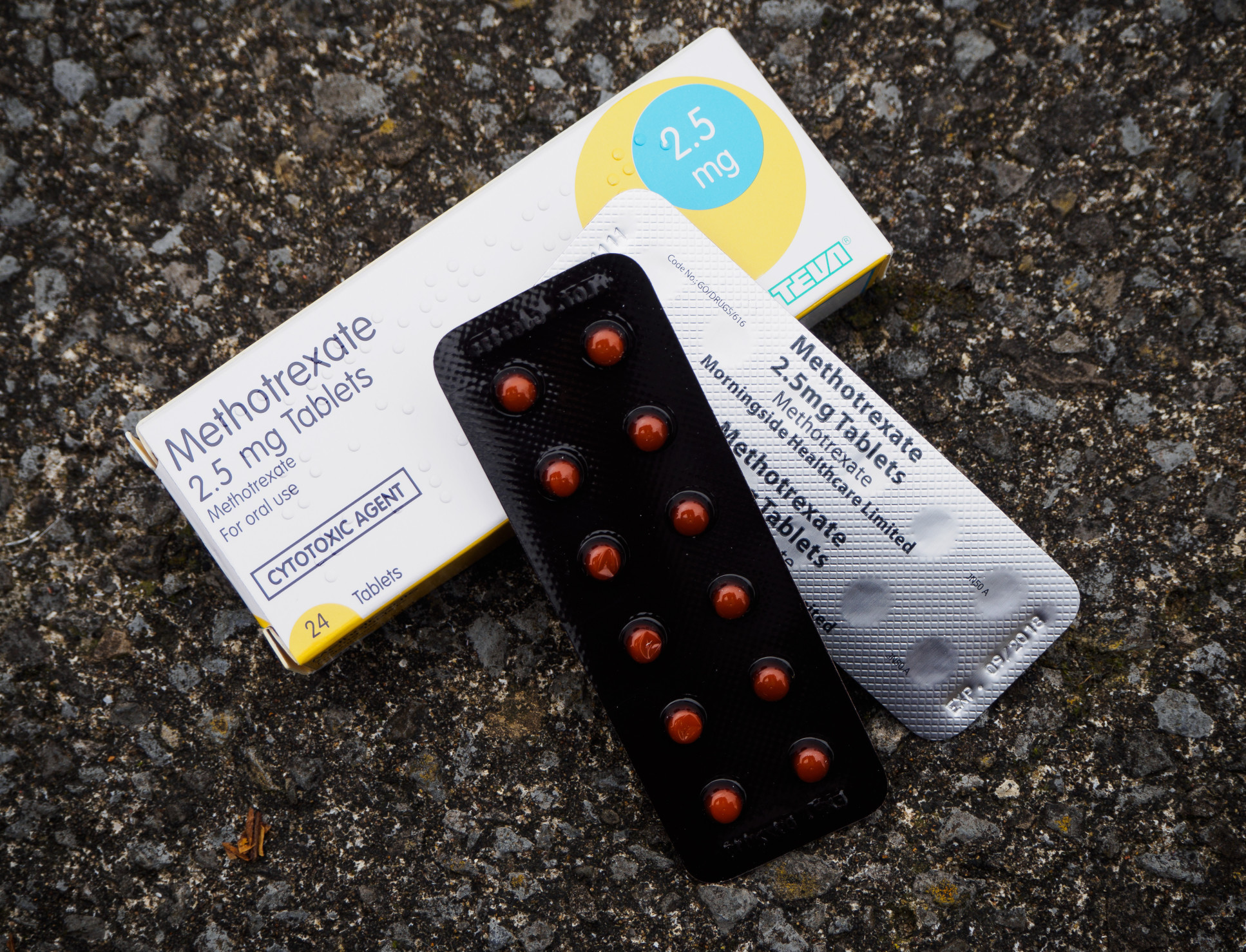
For the Vaccine Response On Off Methotrexate (VROOM) trial, 127 participants were told to stop using methotrexate for two weeks after their third COVID-19 vaccine dose, while another 127 participants were told to continue using it as usual. Although the study, which is published in The Lancet Respiratory Medicine, had planned to recruit 560 patients, recruitment was stopped early by the independent study oversight committee when interim results from the first 254 participants showed such a clear result. Antibody response doublingWhen the researchers compared the antibody levels resulting from vaccination between the two groups at four weeks and 12 weeks, they found that the level was more than two-fold higher in the group that had suspended methotrexate use. The level of the antibody, which blocks the virus from infecting cells, reflects the strength of the immune system’s antibody response. The researchers found that there was a worsening of disease control at week four in the group that stopped using the drug, but that this had normalised by week 12 and there was no adverse impact on the quality of life of the patients. "Implementing these results could improve the protection provided by boosters against COVID-19 for millions of people living with these conditions." Professor Abhishek Abhishek Professor of Rheumatology at the University of Nottingham Chief Investigator Professor Abhishek Abhishek, from the University of Nottingham, said: “We are extremely pleased with the initial results of the VROOM trial. There was a doubling of the antibody response in patients who held off on taking methotrexate for two weeks. “The improvement in antibody response was maintained over a 3-month period. There was a short-term increase in risk of flare-up of inflammatory conditions. However, most could be self-managed.” He added: “Implementing these results could improve the protection provided by boosters against COVID-19 for millions of people living with these conditions. “COVID-19 has left them vulnerable to serious illness, whilst still having to live with the painful and troubling effects of their conditions. We hope this evidence is the next step in helping them with their lives going forward.” Participants in the VROOM trial have also been invited to participate in an additional visit six months after their vaccination date to assess the durability of their immune response. The study was funded by the National Institute for Health Research and the Medical Research Council. It was run by the Oxford Clinical Trials Research Unit. ‘Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial’ by Abhishek Abhishek et al. is published in The Lancet Respiratory Medicine. |
| URL | 查看原文 |
| 来源平台 | Imperial College London |
| 文献类型 | 新闻 |
| 条目标识符 | http://119.78.100.173/C666/handle/2XK7JSWQ/352520 |
| 专题 | 资源环境科学 |
| 推荐引用方式 GB/T 7714 | admin. Interrupting immune-suppressing treatment can boost COVID vaccine response. 2022. |
| 条目包含的文件 | 条目无相关文件。 | |||||
| 个性服务 |
| 推荐该条目 |
| 保存到收藏夹 |
| 查看访问统计 |
| 导出为Endnote文件 |
| 谷歌学术 |
| 谷歌学术中相似的文章 |
| [admin]的文章 |
| 百度学术 |
| 百度学术中相似的文章 |
| [admin]的文章 |
| 必应学术 |
| 必应学术中相似的文章 |
| [admin]的文章 |
| 相关权益政策 |
| 暂无数据 |
| 收藏/分享 |
除非特别说明,本系统中所有内容都受版权保护,并保留所有权利。
修改评论